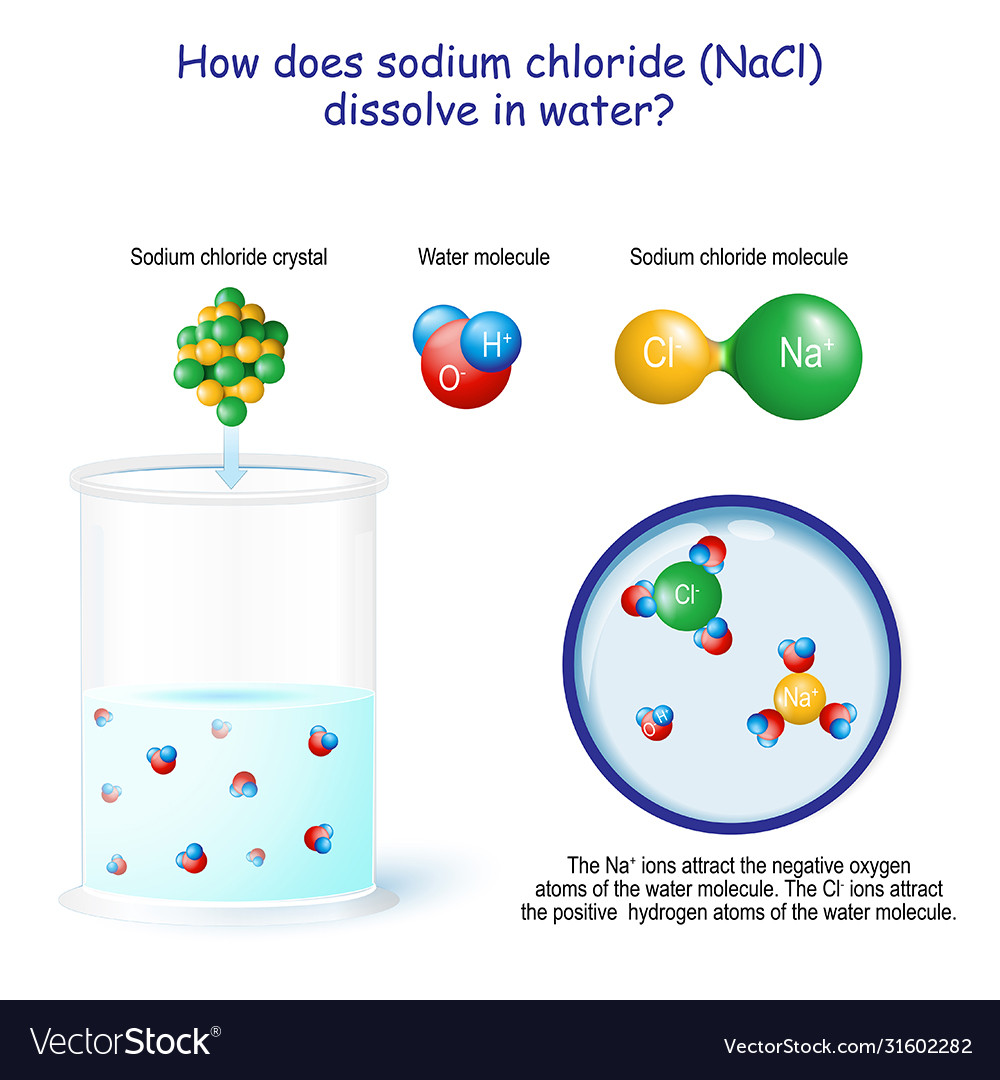
Solid Licl Dissolves In Water Because . solution solid licl is added to water. solid licl is added to water. when solid licl is added to water it dissolves because a. The li+ ions are attracted to the 1) oxygen atom (δ −) of water. The li+ ions are attracted to the oxygen atom (δ−) of water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solid licl dissolves in water because: (select all that apply) a. solid licl dissolves in water because: because water is a polar substance, the interactions between both li + and cl − ions and water should be favorable and strong.
from www.vectorstock.com
when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. because water is a polar substance, the interactions between both li + and cl − ions and water should be favorable and strong. solid licl dissolves in water because: (select all that apply) a. The li+ ions are attracted to the oxygen atom (δ−) of water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solution solid licl is added to water. solid licl is added to water. solid licl dissolves in water because: when solid licl is added to water it dissolves because a.
How does sodium chloride nacl dissolve in water Vector Image
Solid Licl Dissolves In Water Because when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solid licl dissolves in water because: when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solution solid licl is added to water. because water is a polar substance, the interactions between both li + and cl − ions and water should be favorable and strong. solid licl is added to water. (select all that apply) a. The li+ ions are attracted to the oxygen atom (δ−) of water. The li+ ions are attracted to the 1) oxygen atom (δ −) of water. when solid licl is added to water it dissolves because a. solid licl dissolves in water because:
From www.twinkl.es
What is Dissolving? Answered Twinkl Teaching Wiki Solid Licl Dissolves In Water Because when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solid licl dissolves in water because: when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solid licl is added to water. The li+ ions are attracted to. Solid Licl Dissolves In Water Because.
From www.researchgate.net
(a) Schematic phase diagram of waterLiCl mixtures. 228 Locally Solid Licl Dissolves In Water Because solid licl dissolves in water because: The li+ ions are attracted to the 1) oxygen atom (δ −) of water. because water is a polar substance, the interactions between both li + and cl − ions and water should be favorable and strong. The li+ ions are attracted to the oxygen atom (δ−) of water. solution solid. Solid Licl Dissolves In Water Because.
From exopfjfqd.blob.core.windows.net
Liquid That Dissolves Solid Called at Mary Boudreau blog Solid Licl Dissolves In Water Because solid licl dissolves in water because: The li+ ions are attracted to the 1) oxygen atom (δ −) of water. solution solid licl is added to water. because water is a polar substance, the interactions between both li + and cl − ions and water should be favorable and strong. solid licl is added to water.. Solid Licl Dissolves In Water Because.
From slideplayer.com
תמיסה היא תערובת הומוגנית המורכבת מממס ומומס ppt download Solid Licl Dissolves In Water Because solid licl dissolves in water because: when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The li+ ions are attracted to the oxygen atom (δ−) of water. solid licl dissolves in water because: when ionic compounds dissolve in water, the ions in the solid separate and. Solid Licl Dissolves In Water Because.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures Solid Licl Dissolves In Water Because (select all that apply) a. solid licl dissolves in water because: when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The li+ ions are attracted to the 1). Solid Licl Dissolves In Water Because.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID Solid Licl Dissolves In Water Because solid licl dissolves in water because: The li+ ions are attracted to the 1) oxygen atom (δ −) of water. (select all that apply) a. solid licl dissolves in water because: solution solid licl is added to water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. Solid Licl Dissolves In Water Because.
From www.slideserve.com
PPT Solubility and Solutions PowerPoint Presentation, free download Solid Licl Dissolves In Water Because when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solid licl is added to water. (select all that apply) a. because water is a polar substance, the interactions between both li + and cl − ions and water should be favorable and strong. when solid licl. Solid Licl Dissolves In Water Because.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Solid Licl Dissolves In Water Because solid licl is added to water. (select all that apply) a. because water is a polar substance, the interactions between both li + and cl − ions and water should be favorable and strong. when solid licl is added to water it dissolves because a. solid licl dissolves in water because: The li+ ions are attracted. Solid Licl Dissolves In Water Because.
From slideplayer.com
Chapter 7 Solutions 7.1 Solutions ppt download Solid Licl Dissolves In Water Because solid licl is added to water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when solid licl is added to water it dissolves because a. solution solid licl is added to water. solid licl dissolves in water because: solid licl dissolves in water. Solid Licl Dissolves In Water Because.
From www.chegg.com
Solved Solutions Q1 Solid LiCl is added to water. It Solid Licl Dissolves In Water Because solid licl dissolves in water because: (select all that apply) a. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The li+ ions are attracted to the 1) oxygen atom (δ −) of water. solid licl is added to water. The li+ ions are attracted to the. Solid Licl Dissolves In Water Because.
From www.numerade.com
SOLVED LiCl dissolves in water (polar) because water is polar. LiCl is Solid Licl Dissolves In Water Because (select all that apply) a. The li+ ions are attracted to the 1) oxygen atom (δ −) of water. solid licl dissolves in water because: when solid licl is added to water it dissolves because a. solution solid licl is added to water. when ionic compounds dissolve in water, the ions in the solid separate and. Solid Licl Dissolves In Water Because.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image Solid Licl Dissolves In Water Because because water is a polar substance, the interactions between both li + and cl − ions and water should be favorable and strong. solid licl dissolves in water because: when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The li+ ions are attracted to the oxygen atom. Solid Licl Dissolves In Water Because.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Solid Licl Dissolves In Water Because (select all that apply) a. The li+ ions are attracted to the 1) oxygen atom (δ −) of water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solid licl dissolves in water because: solid licl dissolves in water because: because water is a polar substance,. Solid Licl Dissolves In Water Because.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Solid Licl Dissolves In Water Because solid licl is added to water. solid licl dissolves in water because: when solid licl is added to water it dissolves because a. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The li+ ions are attracted to the oxygen atom (δ−) of water. solution. Solid Licl Dissolves In Water Because.
From 2012books.lardbucket.org
Reactions in Aqueous Solution Solid Licl Dissolves In Water Because solution solid licl is added to water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The li+ ions are attracted to the 1) oxygen atom (δ −) of water. The li+ ions are attracted to the oxygen atom (δ−) of water. when solid licl is added. Solid Licl Dissolves In Water Because.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? Solid Licl Dissolves In Water Because The li+ ions are attracted to the oxygen atom (δ−) of water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. because water is a polar substance, the interactions between both li + and cl − ions and water should be favorable and strong. when ionic compounds. Solid Licl Dissolves In Water Because.
From exopfjfqd.blob.core.windows.net
Liquid That Dissolves Solid Called at Mary Boudreau blog Solid Licl Dissolves In Water Because The li+ ions are attracted to the 1) oxygen atom (δ −) of water. (select all that apply) a. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solid licl dissolves in water because: because water is a polar substance, the interactions between both li + and. Solid Licl Dissolves In Water Because.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Solid Licl Dissolves In Water Because The li+ ions are attracted to the 1) oxygen atom (δ −) of water. solid licl is added to water. solid licl dissolves in water because: solution solid licl is added to water. The li+ ions are attracted to the oxygen atom (δ−) of water. when solid licl is added to water it dissolves because a.. Solid Licl Dissolves In Water Because.